Exploring Adicet’s Novel Allogeneic Gamma Delta CAR T Cell Platform
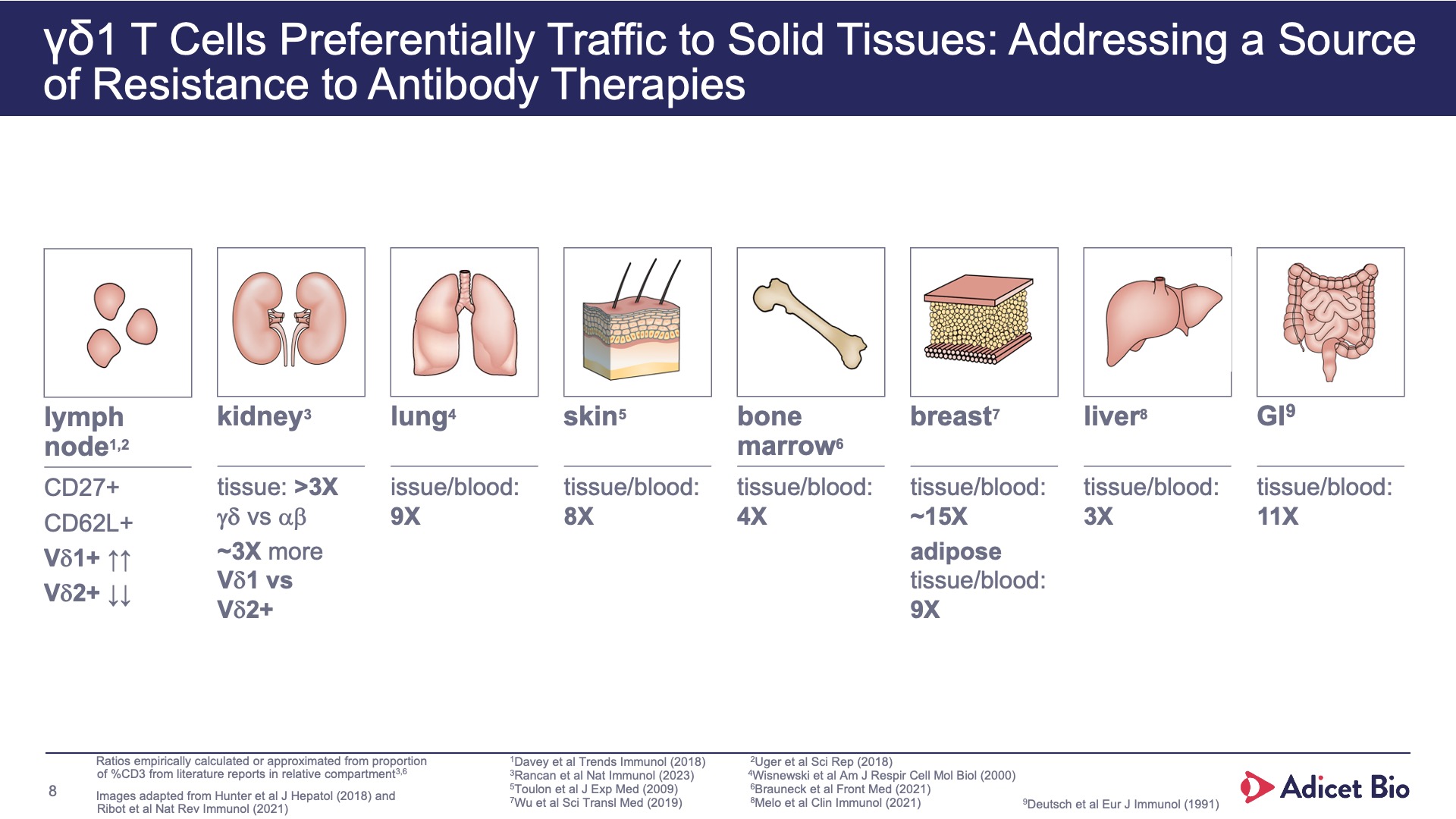
- Adicet’s world class researchers selected gamma delta T cells because they preferentially home to lymphoid tissue and organs, enabling immune reset in peripheral blood and tissue
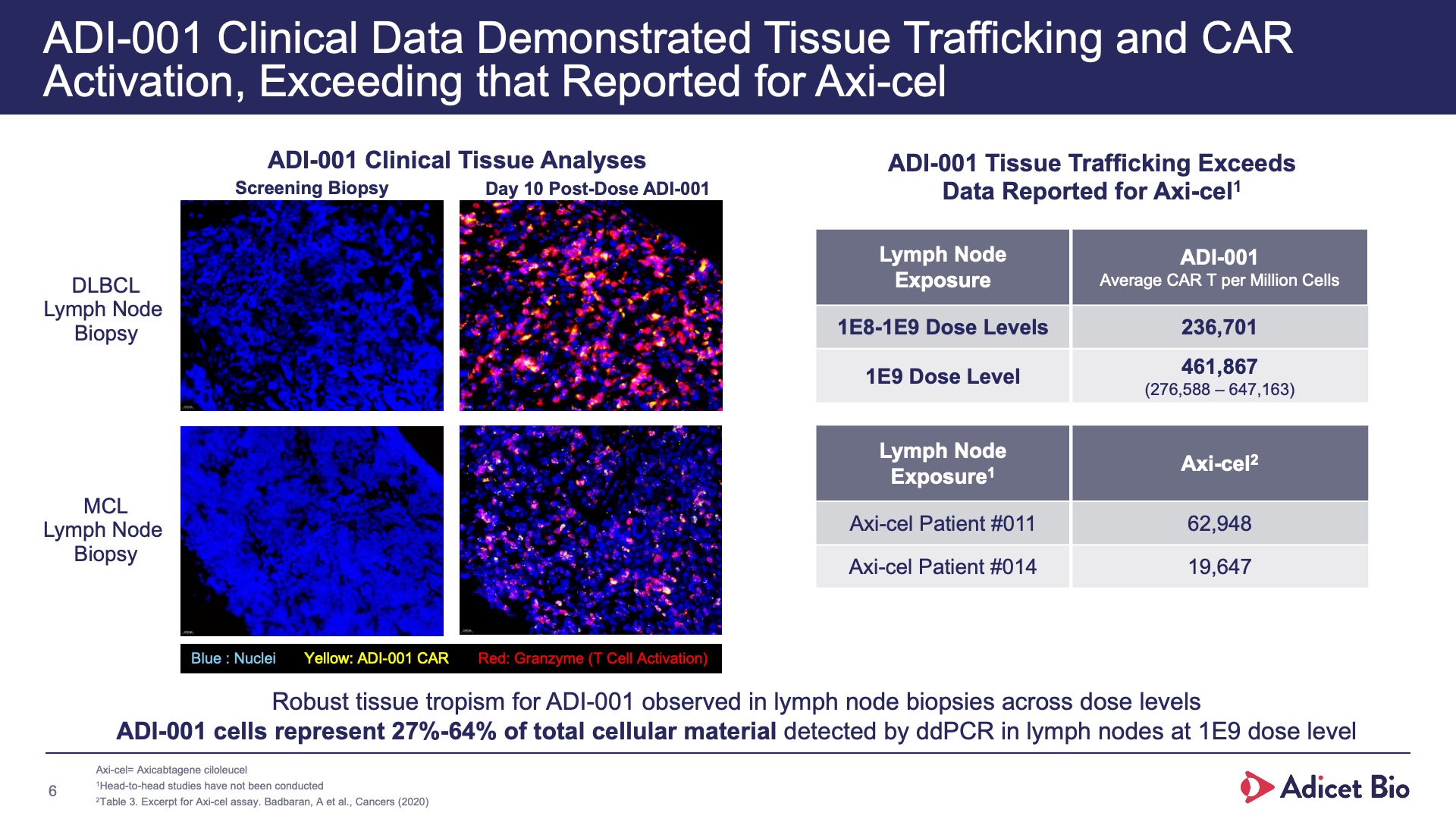
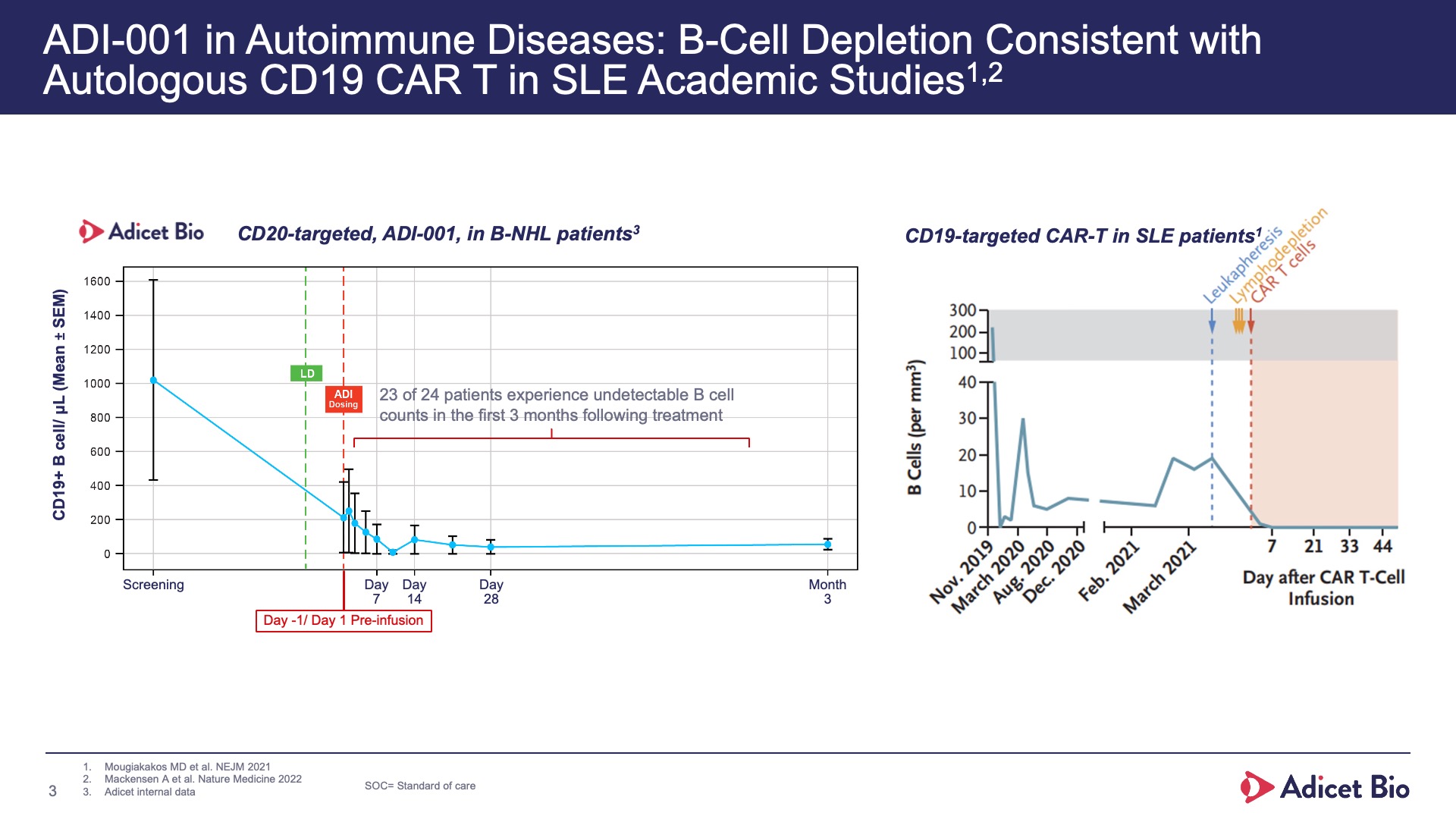
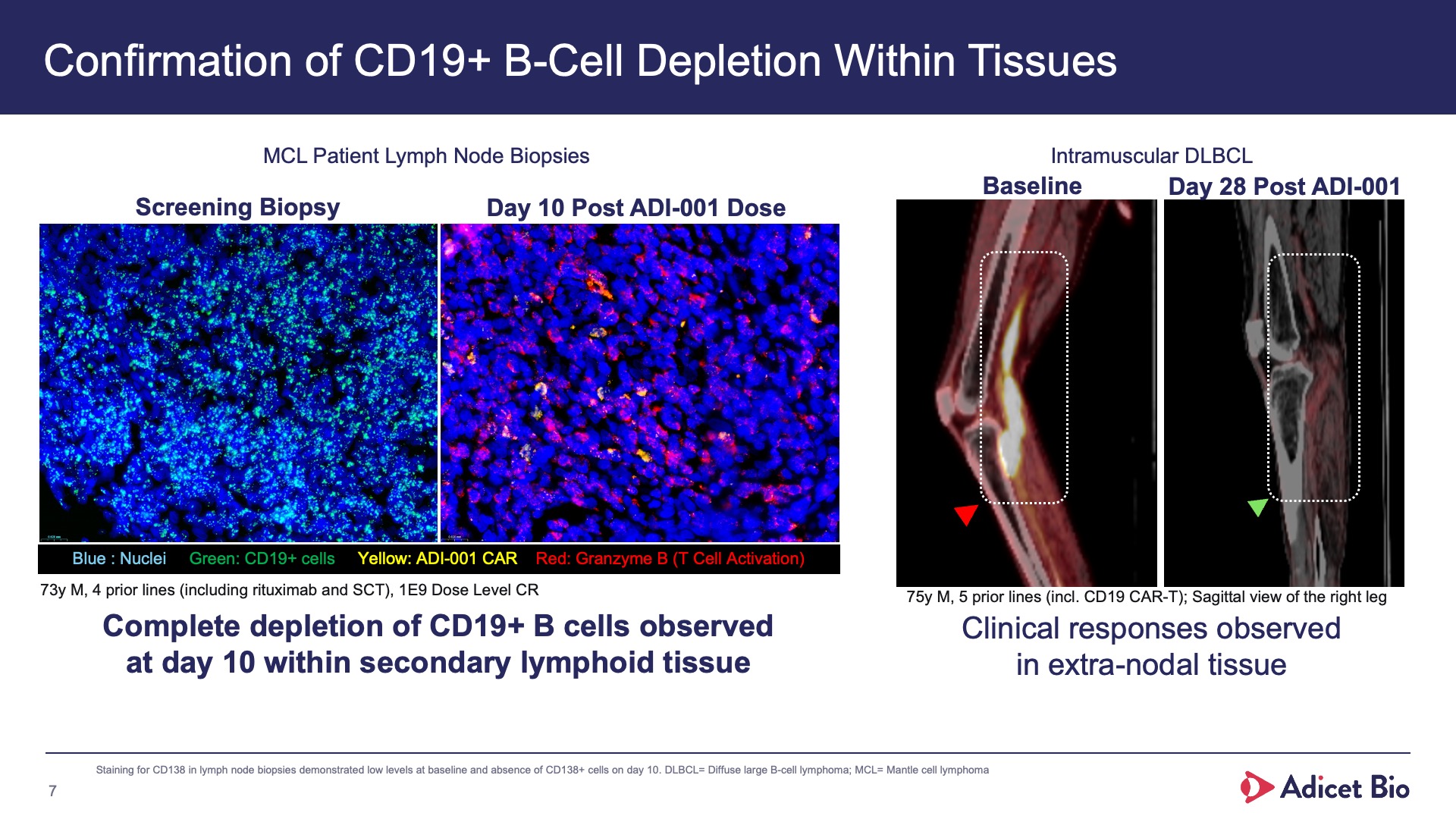
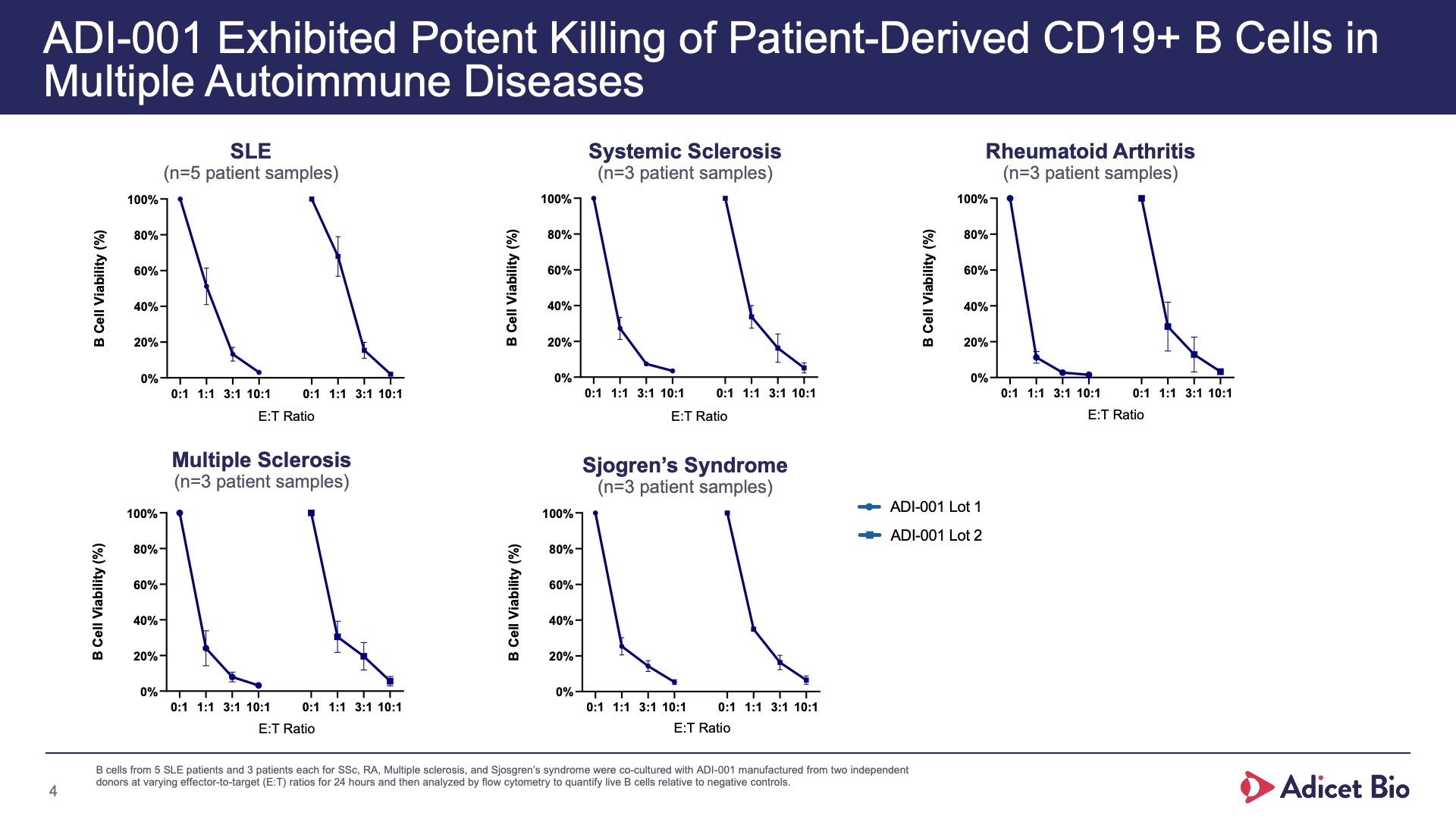
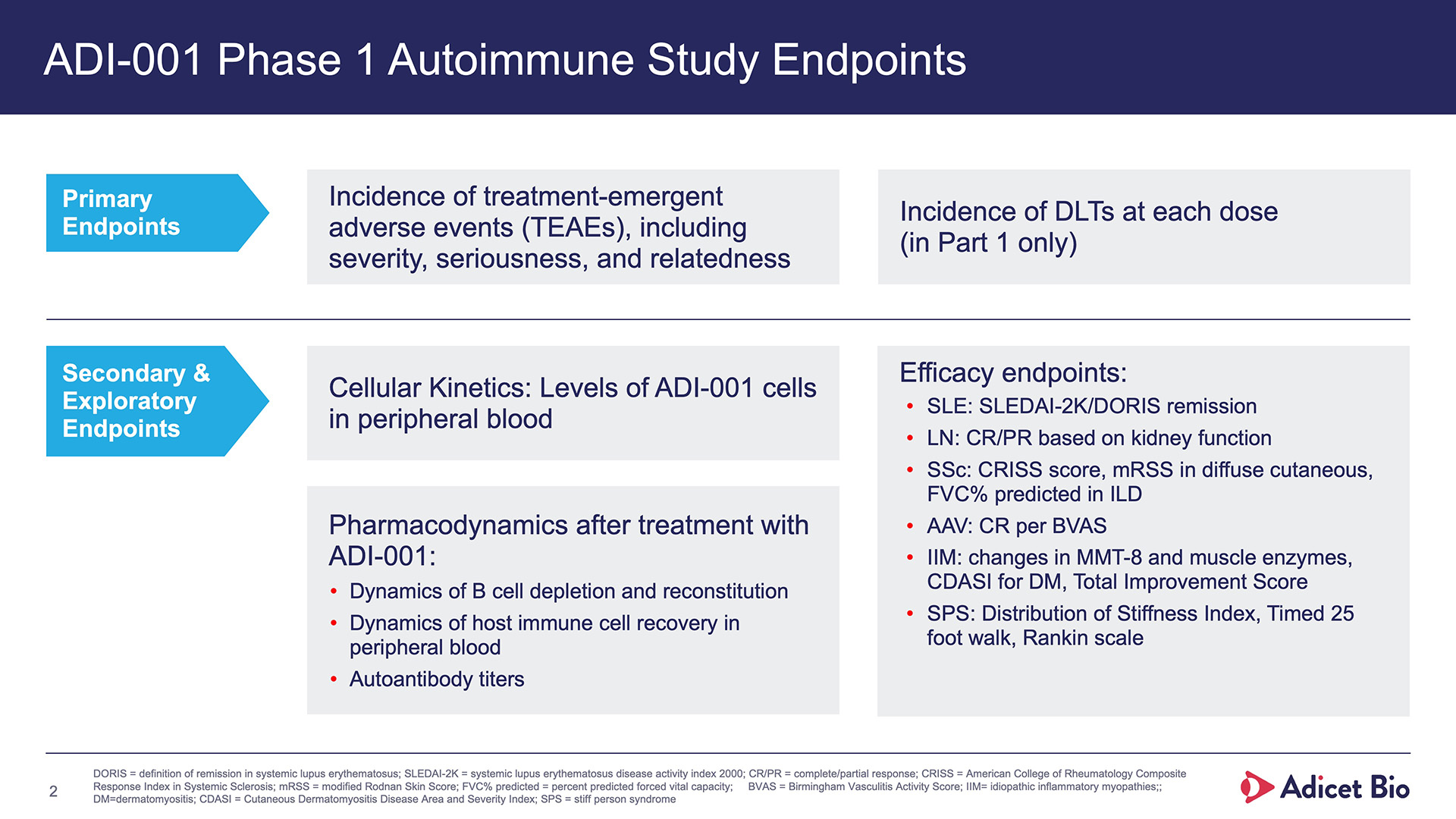
- Adicet clinical data demonstrated complete CD19+ B cell depletion in peripheral blood and secondary lymphoid tissue
- Additionally, data show that targeting CD20 fully depletes the B cell lineage in the peripheral blood, including plasmablasts2
- We’ve eliminated the CD19+ cells with this CD20-targeting CAR T just as well as a CD19-targeting CAR T, by acting at the source of the B cells, depleting the precursors to the plasmablasts.
- From data in our oncology program, no risk of T cell malignancies and reduced risk of CRS and ICANS compared to alpha beta T cell approaches
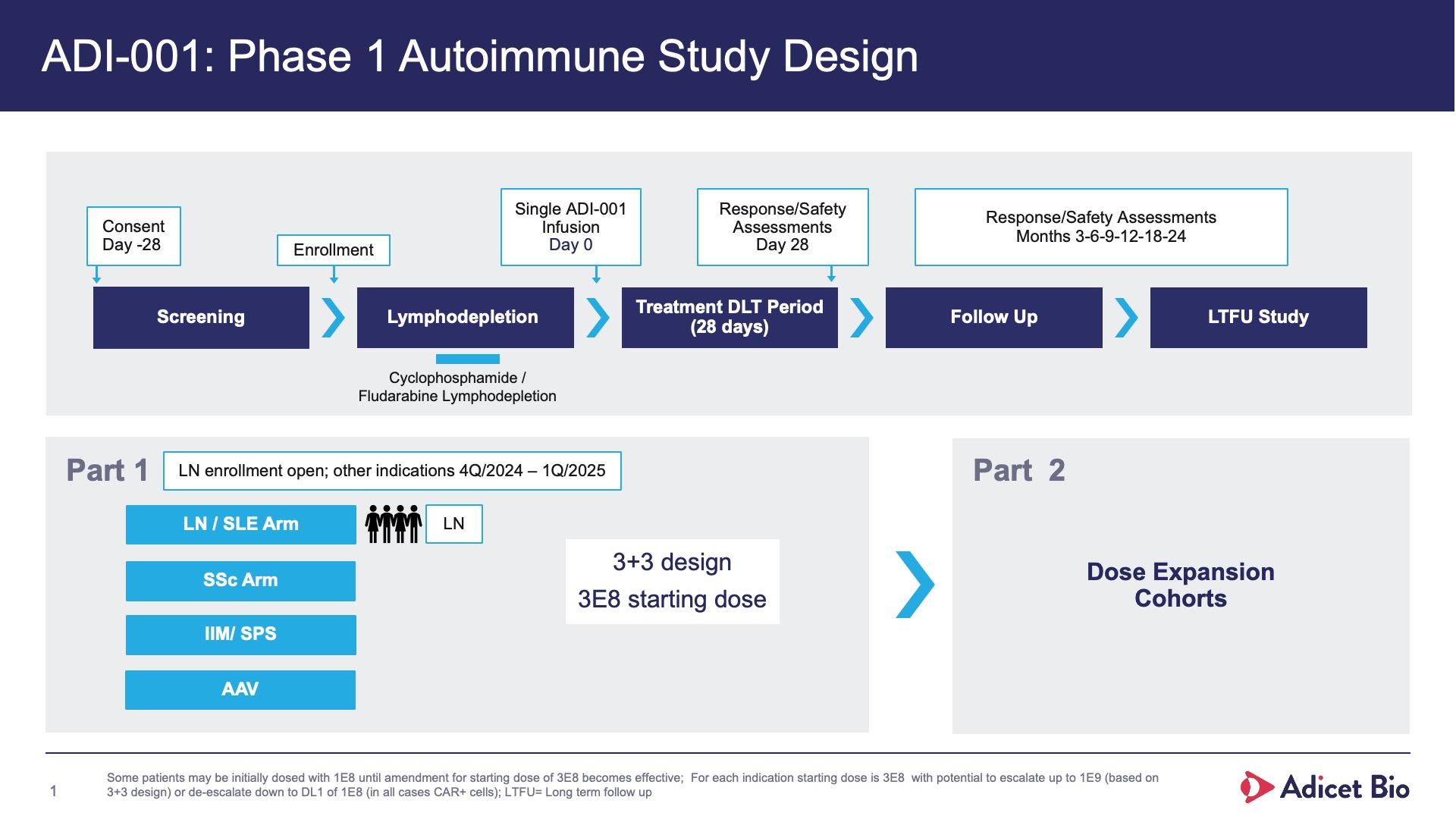
- Because it is allogeneic, no leukapheresis or bridging period for manufacturing is required and the cell therapy can be given on demand, i.e., off-the-shelf.
With its safety profile, robust tissue trafficking and complete B cell depletion in peripheral blood and secondary lymphoid tissue, ADI-001 is uniquely designed to bring sustained, treatment-free remissions for patients with various autoimmune conditions.
One dose of off-the-shelf ADI-001 has the potential to reset the immune system so patients can achieve sustained disease remission, no longer requiring the ongoing challenges associated with many existing therapeutic options.