The current engineered autologous alpha beta T cell products have shown significant efficacy in hematological malignancies and encouraging efficacy in several autoimmune diseases. However, there remains a significant need for efficacious and safe cell therapies that can be provided off-the-shelf (i.e. on demand) to patients suffering from autoimmune diseases, solid tumors and hematological malignancies.
The cell platform and novel targeting approaches Adicet is developing are aimed at harnessing the advantages of gamma delta T cells. The Company believes that gamma delta T cells may have a superior potential compared to alpha beta T cells to be active in both autoimmune diseases, solid tumors and hematological malignancies.
Gamma Delta T Cells
Our lead product candidate, ADI-001, is an investigational allogeneic gamma delta CAR T cell therapy that targets B-cells via an anti-CD20 CAR. ADI-001 is developed as a potential treatment for B cell-mediated autoimmune diseases.
Autoimmune Diseases
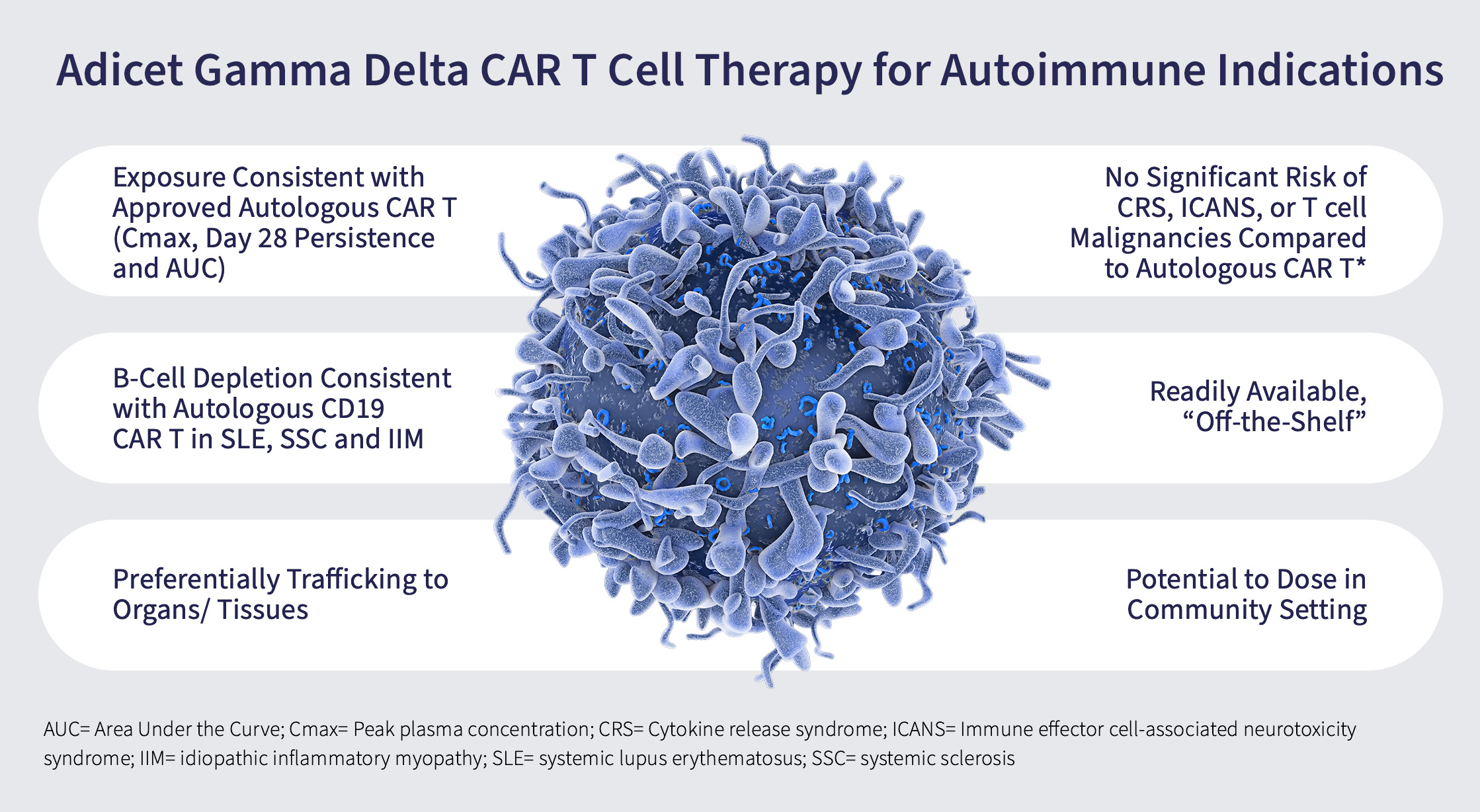
Potential Advantages of Gamma Delta T cells in Autoimmune Diseases
Drug Exposure: Adicet’s off-the-shelf gamma-delta 1 CAR T cell therapies are unique as they may provide patients with the exposure of a cell therapy that is consistent with an autologous CAR T.
- This means the patient may receive enough of the cells to achieve the desired therapeutic effect.
Immune reset: B-cell depletion (or depopulation) data from the ADI-001 trial in cancer patients mirrors B-cell depletion by autologous CD19 CAR-T in several autoimmune diseases, including systemic lupus erythematosus (SLE), systemic sclerosis and idiopathic inflammatory myopathy.
- Depopulating B-cells is a key to treating various autoimmune diseases. ADI-001 is designed to target and deplete or depopulate B cells in kidneys and other organs which is highly desirable in autoimmune diseases.
Homing to tissues: An important advantage of gamma-delta 1 T cells is that they naturally home or traffic to tissues. This may be important in many autoimmune diseases where patients experience damage to organs in their body. Being able to depopulate errant B cells located in peripheral tissues may better enable an effective reset of the immune system, a highly desirable outcome in the treatment of autoimmune diseases.
- Many cell therapies work well in the blood but are suboptimal in their ability to exert activity in tissues or various organs. Gamma delta 1 T cells have an affinity for tissues, with an ability to target the source of disease.
Safety: ADI-001 has shown to be well-tolerated, with no significant incidence of CRS (cytokine release syndrome) or ICANS (immune effector cell-associated neurotoxicity syndrome), and lower risk of T cell malignancies than seen with autologous CAR T cell therapies.
Cancer

Potential Advantages of Gamma Delta T cells in Cancer
Adaptive & Innate: Gamma delta T cells have unique attributes that Adicet believes make them especially well-suited for cancer therapy. The unique features of these cells combine adaptive (gamma delta TCR-mediated) and innate (NK-cell like) immunity to specifically recognize and eliminate tumor cells while sparing normal, healthy cells.
MHC-Independent: Unlike alpha beta T cells, gamma delta T cells perform their tumor killing function in an MHC-independent* manner and thus can be used in an allogeneic setting without the risk of causing Graft vs Host Disease (GvHD).
Tissue-Homing: Additionally, gamma delta T cells perform their immune surveillance by naturally homing to various tissues giving them a superior potential to alpha beta T cells to eradicate solid tumors in tissues. Adicet has demonstrated the cytotoxicity and anti-tumor activity of gamma delta T cells in in vitro and in vivo in mouse models that translated to anti-tumor activity in patients suffering from NHL.
*MHC stands for major histocompatibility complex, which is a group of genes that code for proteins found on the surfaces of cells that help the immune system recognize foreign substances. Because they are MHC-independent, the gamma delta T cells being administered to patients from healthy donors are not seen as foreign and are therefore accepted so they can treat diseases as needed.
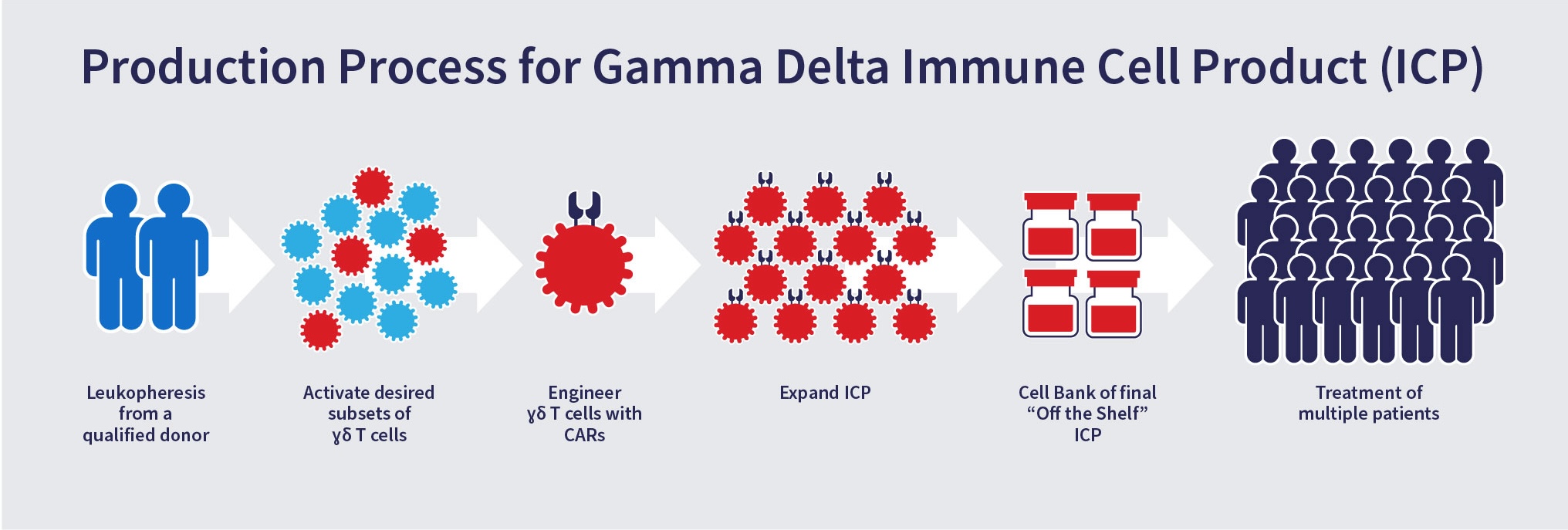
Proprietary Manufacturing and Cell Platform Process
To date, the use of gamma delta T cells has been limited because they represent only a small fraction of the peripheral blood mononuclear cells (1-5%). Adicet has developed a proprietary and robust manufacturing process to activate and expand different subsets of gamma delta T cells to numbers sufficient for utilization in the clinical setting.
The activation, engineering and expansion of our gamma delta T cells allows for generation of cell therapy products that can be used to treat many patients.